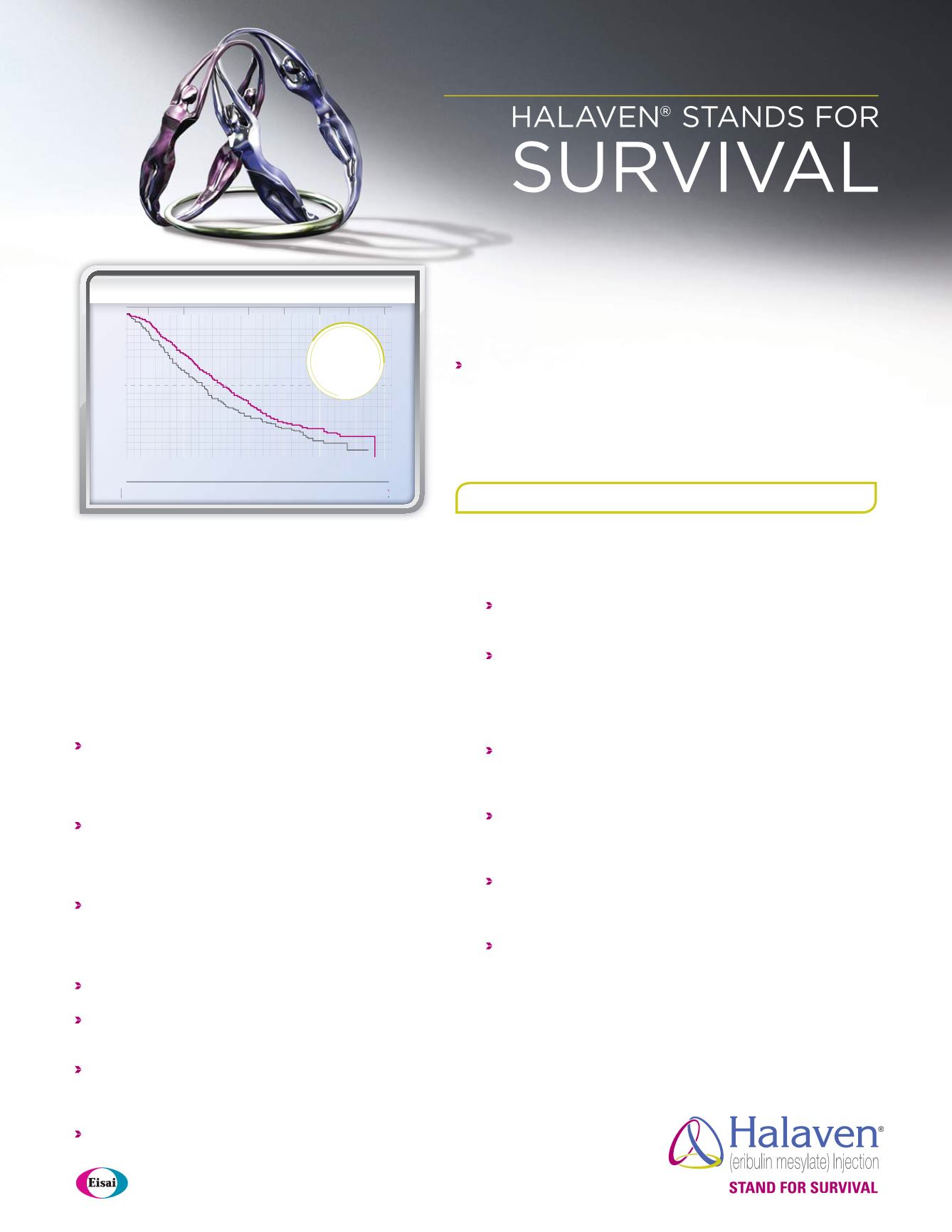
TPC
HALAVEN
Number of
patients at risk
508
406
274
142
54
11
0
254
178
106
61
26
5
0
1.0
0.9
0.8
0.7
0.6
0.5
0.4
0.3
0.2
0.1
0.0
0
6
12
18
24
30
36
TIME (MONTHS)
PROPORTION OF PATIENTS ALIVE
10.6
(9.2, 12.0)
Deaths=203
Treatmentof
Physician’sChoice
(n=254)
13.2
(12.1, 14.4)
Deaths=386
HALAVEN
(n=508)
25%
(2.6 months)
INCREASE
IN MEDIAN OS
UPDATED OS ANALYSIS (UNPLANNED)
:
MEDIAN OS, MONTHS (95% CI)
1,3,a
Indication
HALAVEN (eribulin mesylate) is indicated for the treatment of
patients with metastatic breast cancer who have previously
received at least two chemotherapeutic regimens for the
treatment of metastatic disease. Prior therapy should have
included an anthracycline and a taxane in either the adjuvant or
metastatic setting.
Important Safety Information
Neutropenia
Monitor complete blood counts prior to each dose, and increase
the frequency of monitoring in patients who develop Grade 3
or 4 cytopenias. Delay administration and reduce subsequent
doses in patients who experience febrile neutropenia or Grade 4
neutropenia lasting longer than 7 days
Severe neutropenia (ANC <500/mm
3
) lasting more than 1 week
occurred in 12% (62/503) of patients. Patients with elevated
liver enzymes >3 × ULN and bilirubin >1.5 × ULN experienced
a higher incidence of Grade 4 neutropenia and febrile
neutropenia than patients with normal levels
Grade 3 and Grade 4 neutropenia occurred in 28% and 29%,
respectively, of patients who received HALAVEN. Febrile
neutropenia occurred in 5% of patients and two patients
(0.4%) died from complications
Peripheral Neuropathy
Patients should be monitored closely for signs of peripheral
motor and sensory neuropathy
Grade 3 peripheral neuropathy occurred in 8% of patients, and
Grade 4 in 0.4% of patients who received HALAVEN. Delay
administration of HALAVEN until resolution to Grade 2 or less
Neuropathy lasting more than 1 year occurred in 5% of
patients.Twenty-two percent of patients developed a new or
worsening neuropathy that had not recovered within a median
follow-up duration of 269 days (range 25-662 days)
Peripheral neuropathy (5%) was the most common adverse
reaction resulting in discontinuation
Pregnancy Category D
HALAVEN is expected to cause fetal harm when administered to
a pregnant woman and patients should be advised of these risks
QT Prolongation
In an uncontrolled ECG study in 26 patients, QT prolongation
was observed on Day 8, independent of eribulin concentration,
with no prolongation on Day 1. ECG monitoring is recommended
for patients with congestive heart failure; bradyarrhythmias;
concomitant use of drugs that prolong QT interval, including
Class Ia and III antiarrhythmics; and electrolyte abnormalities
Correct hypokalemia or hypomagnesemia prior to initiating
HALAVEN and monitor electrolytes periodically during
therapy. Avoid in patients with congenital long QT syndrome
Hepatic and Renal Impairment
For patients with mild (Child-Pugh A) or moderate (Child-
Pugh B) hepatic and/or moderate (CrCl 30-50 mL/min) renal
impairment, a reduction in starting dose is recommended
Most Common Adverse Reactions
Most common adverse reactions (≥25%) reported in patients
receiving HALAVEN were neutropenia (82%), anemia (58%),
asthenia/fatigue (54%), alopecia (45%), peripheral neuropathy
(35%), nausea (35%), and constipation (25%)
The most common serious adverse reactions reported in
patients receiving HALAVEN were febrile neutropenia (4%)
and neutropenia (2%)
Please see accompanying brief summary of HALAVEN full
Prescribing Information.
References: 1.
HALAVEN [package insert]. Woodcliff Lake, NJ: Eisai Inc; 2014.
2.
Towle MJ, et al.
Cancer Res.
2001;61(3):1013-1021.
3.
Cortes J, et al.
Lancet.
2011;377(9769):914-923.
4.
Saad ED, et al.
J Clin Oncol.
2010;28(11):1958-1962.
5.
Sparano JA, et al.
J Clin Oncol.
2010;28(20):3256-3263.
HALAVEN
®
is a registered trademark used by Eisai Inc. under license from Eisai R&D Management Co., Ltd.
© 2014 Eisai Inc.
All rights reserved.
Printed in USA/November 2014
HALA0783
For patients who stand up to metastatic breast cancer
For health care teams that stand up for those patients
The ability to extend life with the
first agent in the halichondrin class
1,2
Results from an updated, unplanned survival analysis of the Phase III, randomized, open-label, multicenter, multinational Eisai Metastatic Breast Cancer Study Assessing Physician’s Choice versus E7389 (Eribulin) (EMBRACE)
trial of HALAVEN versus TPC in patients with MBC (N=762), conducted when 77% of events (deaths) had been observed. The primary endpoint was OS. Patients were randomized (2:1) to receive either HALAVEN 1.4 mg/m
2
intravenously for 2 to 5 minutes on Days 1 and 8 of a 21-day cycle, or any single-agent therapy, selected prior to randomization. At baseline, all patients had received ≥2 prior chemotherapeutic regimens for metastatic disease
and demonstrated disease progression within 6 months of their last chemotherapeutic regimen. All patients received prior anthracycline- and taxane-based chemotherapy, unless contraindicated. Therapies in the TPC arm
consisted of 97% chemotherapy (26% vinorelbine, 18% gemcitabine, 18% capecitabine, 16% taxanes [included paclitaxel, docetaxel, nab-paclitaxel, and ixabepilone], 9% anthracyclines, 10% other chemotherapy), and 3% hormonal therapy.
The first and only single agent that significantly extended
overall survival in third-line MBC
3-5
The updated OS analysis was consistent with the
primary analysis
1
The primary analysis, conducted when ~50% of events (deaths) had
been observed, demonstrated a median OS for HALAVEN vsTPC of
13.1 months (95% CI: 11.8, 14.3) vs 10.6 months (95% CI: 9.3, 12.5),
hazard ratio=0.81 (95% CI: 0.66, 0.99) (
P
=0.041)
1,3
OS=overall survival; CI=confidence interval; MBC=metastatic breast cancer; TPC=Treatment of Physician’s Choice.
a
Conducted in the intent-to-treat population.
Visit


